News
A third type of PETase from the marine Halopseudomonas lineage
17.09.2025
Onur Turak, Andreas Gagsteiger, Ashank Upadhyay, Mark Kriegel, Peter Salein, Stefanie Böhnke-Brandt, Seema Agarwal, Erik Borchert, Birte Höcker
Protein Science, 2025, https://doi.org/10.1002/pro.70305
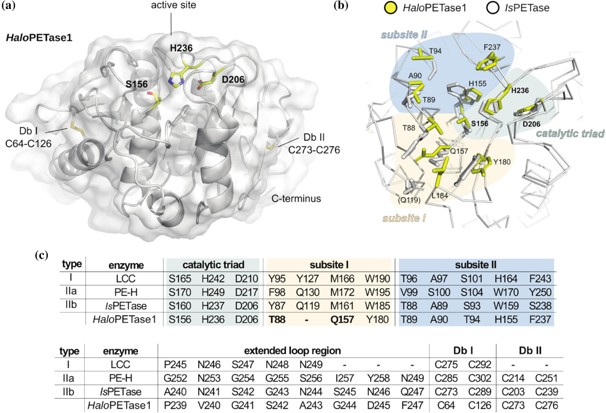
The enzymatic degradation of polyethylene terephthalate (PET) offers a sustainable solution for PET recycling. Over the past two decades, more than 100 PETases have been characterized, primarily exhibiting similar sequences and structures. Here, we report PET-degrading α/β hydrolases, including HaloPETase1 from the marine Halopseudomonas lineage, thereby extending the narrow sequence space by novel features at the active site. The crystal structure of HaloPETase1 was determined to a resolution of 1.16 Å, revealing a unique active site architecture and a lack of the canonical π-stacking clamp found in PETases so far. Further, variations in active site composition and loop structures were observed. Additionally, we found five more enzymes from the same lineage, two of which have a high similarity to type IIa bacterial PETases, while the other three resemble HaloPETase1. All these enzymes exhibited high salt tolerance ranging from 2.5 to 5 M NaCl, leading to higher total product releases upon PET degradation at 40 or 50°C. Based on these findings, we propose an extension of the existing PETase classification system to include type III PETases.