News
Evaluating Polymerization Methods and Deprotection Strategies for Making Water Soluble Poly(acrylic acid) with Hydrolyzable Breaking Points
02.04.2025
Sophia B. Däbritz, Kira Neubauer, Christian Kropf, Seema Agarwal
Macromolecular Chemistry and Physics, 2025, https://doi.org/10.1002/macp.202500080
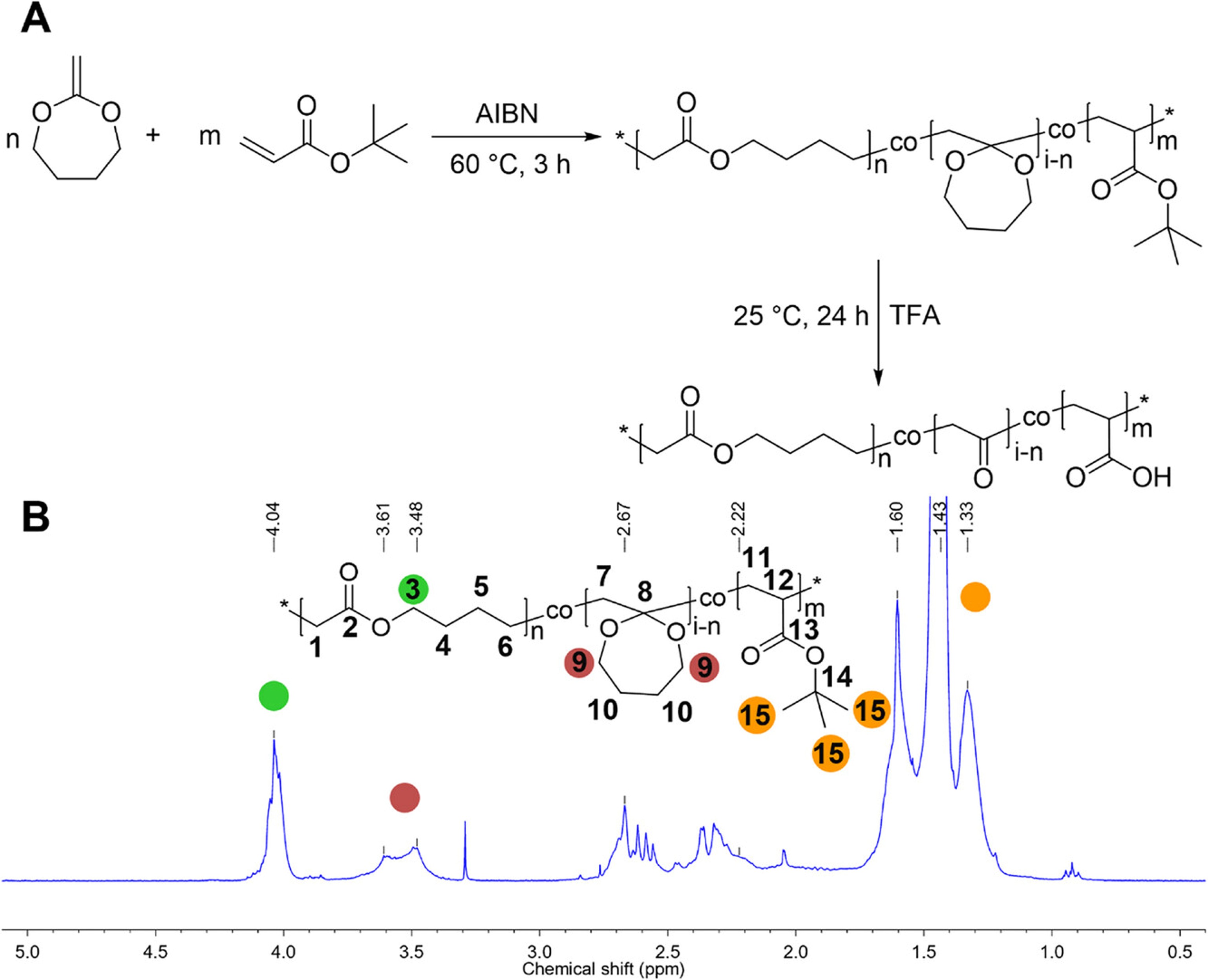
Poly(acrylic acid) (PAA) is a hydrophilic polymer widely utilized in various everyday applications, but it may persist in the environment due to its stable carbon-carbon (C-C) backbone. This work presents a detailed comparative study of introducing hydrolyzable ester breaking points into the PAA backbone using different radical copolymerization methods (bulk versus solvent and batch versus semi-batch) with varied feed ratios of tert-butyl acrylate (tBA) and 2-methylene-1,3-dioxepane (MDO) followed by the investigation of the removal of t-Bu group for getting free acid functionality in copolymers under different conditions. A detailed comparison of polymerization approaches (bulk versus solution, batch versus semi-batch) revealed that solution polymerization at 100 °C with tert-butyl peroxide provided high ring-opening efficiency (71%) and uniform molecular weight distribution. The study optimized deprotection processes for tBA to acrylic acid, achieving complete hydrolysis under mild conditions using 5 equivalents of trifluoroacetic acid in dichloromethane. The resultant polymers displayed pH and temperature dependent solubility and significant degradation under alkaline conditions, with the formation of oligomers (400–700 Da for 35% MDO content) suitable for microbial assimilation. These findings highlight a scalable pathway for creating environmentally degradable PAA alternatives with tailored properties for functional applications.