News
Investigation of the Thermal Stability of Proteinase K for the Melt Processing of Poly(L-lactide)
03.11.2022
Chengzhang Xu, Alexander Battig, Bernhard Schartel, Renée Siegel, Jürgen Senker, Inge von der Forst, Carlo Unverzagt, Seema Agarwal, Andreas Möglich, and Andreas Greiner
Biomacromolecules 2022 https://doi.org/10.1021/acs.biomac.2c01008
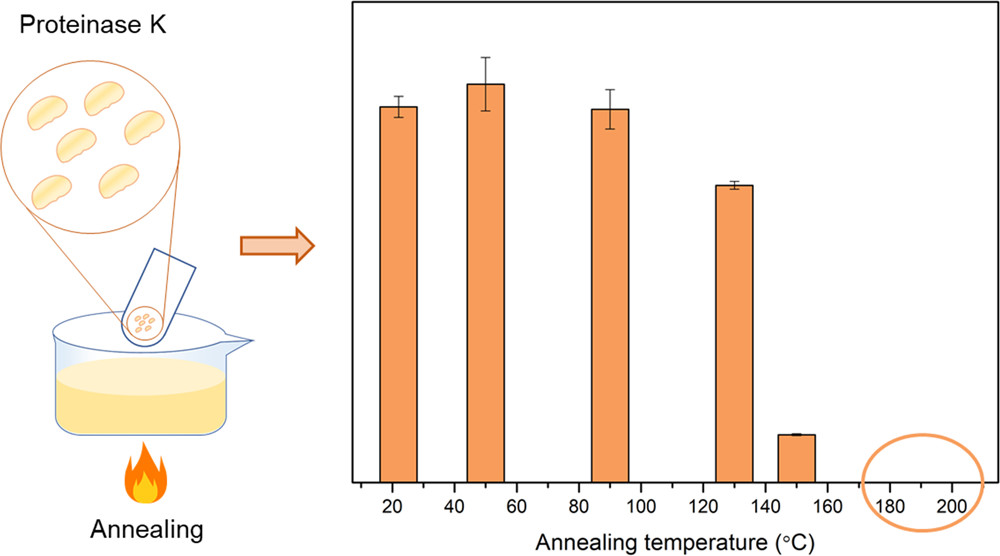
The enzymatic degradation of aliphatic polyesters offers unique opportunities for various use cases in materials science. Although evidently desirable, the implementation of enzymes in technical applications of polyesters is generally challenging due to the thermal lability of enzymes. To prospectively overcome this intrinsic limitation, we here explored the thermal stability of proteinase K at conditions applicable for polymer melt processing, given that this hydrolytic enzyme is well established for its ability to degrade poly(L-lactide) (PLLA). Using assorted spectroscopic methods and enzymatic assays, we investigated the effects of high temperatures on the structure and specific activity of proteinase K. Whereas in solution, irreversible unfolding occurred at temperatures above 75–80 °C, in the dry, bulk state, proteinase K withstood prolonged incubation at elevated temperatures. Unexpectedly little activity loss occurred during incubation at up to 130 °C, and intermediate levels of catalytic activity were preserved at up to 150 °C. The resistance of bulk proteinase K to thermal treatment was slightly enhanced by absorption into polyacrylamide (PAM) particles. Under these conditions, after 5 min at a temperature of 200 °C, which is required for the melt processing of PLLA, proteinase K was not completely denatured but retained around 2% enzymatic activity. Our findings reveal that the thermal processing of proteinase K in the dry state is principally feasible, but equally, they also identify needs and prospects for improvement. The experimental pipeline we establish for proteinase K analysis stands to benefit efforts directed to this end. More broadly, our work sheds light on enzymatically degradable polymers and the thermal processing of enzymes, which are of increasing economical and societal relevance.