News
Competition between Hydrolysis and Radical Ring-Opening Polymerization of MDO in Water. Who Makes the Race?
13.01.2023
Benjamin R. Kordes, Laura Ascherl, Christoph Rüdinger, Timo Melchin, and Seema Agarwal
Macromolecules 2023 https://doi.org/10.1021/acs.macromol.2c01653
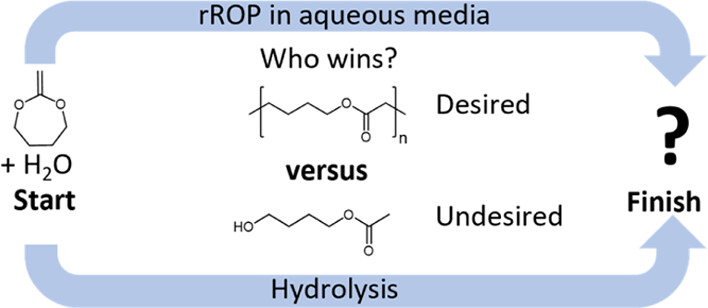
A major challenge in modern society is the reduction of microplastics created by polymers having stable C–C backbones. The chemistry of radical ring-opening copolymerization of cyclic ketene acetals (CKAs) with vinyl monomers in introducing degradable ester units into the C–C backbone is highly promising. Although the corresponding reaction in an aqueous medium should provide biodegradable primary dispersions, the bottleneck is the hydrolytic instability of CKAs. Therefore, in-depth hydrolysis kinetics of CKA (2-methylene-1,3-dioxepane, MDO) at different pH values and temperatures under homogenous and heterogenous conditions are studied to get a “sweet spot” under which emulsion polymerization of MDO might be possible. Depending on the pH, the hydrolysis of MDO undergoes three different mechanisms with slowed hydrolysis kinetics under alkaline conditions. Besides 4-hydroxy-1-butylacetate (4-HBA), other co-hydrolysis products were detected, leading to the autocatalysis effect. The fast MDO hydrolysis during emulsion copolymerization with vinyl acetate led to the formation of polymers with extremely less incorporation of ring-opened MDO units. Degradation tests of the corresponding emulsion copolymers compared with copolymers prepared in solution confirmed the low incorporation ratio of MDO. The results and discussion presented in this work will be a strong guideline for future emulsion copolymerizations of CKAs.